
Researchers started working with methyl glucose as early as the 1960s, seeking alternatives to petroleum-based chemicals that help moisturize and stabilize products in cosmetics and pharmaceuticals. Early patents, especially from Europe and Japan, paved the way for commercial production. Raw glucose from plant sources like corn or wheat built the foundation. Large manufacturers later developed reliable catalyzed methylation processes, which improved purity and cost. Demand picked up in the 1980s with the growth of mild, skin-friendly personal care products. Methyl glucose and its ether and ester derivatives became go-to choices for formulators, a trend that still holds in the modern ingredients market. Larger companies have incorporated traceability tools and batch record systems directly into the supply chain, reflecting increasing scrutiny from regulatory agencies and green chemistry advocates.
Methyl glucose stands out as a creamy-white, odorless solid. In personal experience, handling the powder offers the same stable quality batch after batch, a comfort for any product developer. It dissolves readily in water and forms non-sticky solutions—unlike traditional syrupy polyols. The main role involves working as a humectant and emollient in lotions, creams, shampoos, and conditioners. Surfactant blends can benefit from methyl glucose for improved mildness. In pharmaceuticals, it finds its way into syrups, gels, and topical solutions to regulate viscosity and help stabilize active ingredients. Many methyl glucose-based thickeners and emulsifiers have entered the market under trade names like Glucam and Methocel, each supporting different end-users.
This ingredient shows impressive thermal and chemical stability compared to other simple sugars, so it holds up under high-temperature processing. With a melting range typically in the 95-110°C window, it doesn't decompose or yellow during manufacturing. The molecular formula C7H14O6 gives it a clear place in the family of methylated saccharides. Its high water solubility and low vapor pressure reduce risks in handling. The neutral-to-slightly acidic pH range in solution makes formulation easier, especially for leave-on skin products. Methyl glucose stays non-reactive with most cosmetic actives and is famous for avoiding Maillard reaction browning with amino-containing components.
Almost every reputable supplier lists methyl glucose with a minimum assay over 98%, water content below 1.5%, and ash under 0.1%. Particle size tracks in the 100-250 micron range for powders, although solutions are more common in modern manufacturing to streamline production. For labeling, you’ll see terms like "Methyl Glucose," "Methyl Glucoside," or its INCI names "Methyl Gluceth-10" and "PEG-120 Methyl Glucose Dioleate," depending on the level of ethoxylation or esterification. Food-related applications always require strict compliance to local regulations: for instance, European users check the EINECS/ELINCS lists, and U.S. handlers refer to the FDA’s indirect food additive listings. Safety data sheets detail handling instructions, hazard statements, and storage guidelines directly aligned with globally harmonized system standards.
Methyl glucose usually comes from an acid-catalyzed reaction between glucose and methanol, producing methyl glucoside through a Fischer glycosidation. Industrial processes scale this up with controlled heat and reduced catalyst loading, reducing byproducts. Many plants use continuous reactors to ensure each batch stays within the strict purity window. Downstream, purification includes multiple filtrations, solvent removal, and sometimes chromatography. For special grades like methyl gluceths, manufacturers add ethylene oxide in carefully monitored steps, matching required chain lengths for specific viscosity and solubility profiles. Good operators maintain GMP procedures, and the best avoid cross-contamination with milk or nut derivatives to keep allergens out—a growing demand from regulatory bodies.
Basic methyl glucose works as a starting point for a host of modifications. Its free hydroxyl groups make it ideal for further etherification, esterification, and ethoxylation reactions in bulk. Many surfactants and thickeners in the market today depend on PEG-modified methyl glucose for their positive effects on solubility and gentleness. Manufacturers design high-molecular-weight polymers by linking many methyl glucose units, producing hydrophilic thickeners and stabilizers. Ester derivatives blend better with oils, so these versions show up frequently in lip balms and makeup. On the formulation bench, I've seen this flexibility make tough texture challenges almost effortless—especially versus synthetic alternatives, which can be less forgiving.
Reading labels over the years, you’ve seen methyl glucose called "Methyl D-glucoside," "Methyl alpha-D-glucopyranoside," or "1-O-Methyl-D-glucopyranose." As for commercial blends, names like Glucam E-10, Glucam P-20, Glucquat, and Promidium surfact the shelves, often with different chain lengths and side groups. It's no secret that ingredient lists on popular lotions, facial cleansers, and even baby wipes point to methyl glucoside derivatives—testament to years of safe, widespread use.
Anyone who works in the lab keeps an eye on safety—methyl glucose earns its place thanks to its solid record. Multiple toxicological evaluations mark it as low-risk: skin irritation and eye sting rarely show up, even in concentrated solutions. That said, equipment should still handle high-solids solutions to avoid sticky spills that gum up valves and tanks. Quality system audits stress the need for documented batch traceability, allergen control, and prevention of microbial growth during storage and use. For transport, hazard codes mark it as non-dangerous, so logistical headaches stay minimal. Some plants require closed-system addition for large-scale batching to prevent inhalation of fine powders, along with PPE standards across the board.
In most labs and factories, methyl glucose walks right into hair conditioners, leave-on lotions, facial serums, and gentle baby products. Its ability to hold onto water outperforms traditional glycerin—without the tacky feel or greasiness. In pharmaceuticals, its stable backbone gives active drugs a consistent environment. Mixing with active botanicals or acids comes easy; pH swings don’t send it separating out or building off-colors. Personal experience with methyl glucose as a primary humectant in a lightweight hand cream led to skin hydration without stickiness, which clients consistently praised. Its thickeners extend to oral care and even textile softeners in the household segment, riding on its strong safety profile and renewable raw material credentials. Manufacturers have expanded to use in food additives for improving shelf life, though this rides on regional approval statuses.
Innovation teams today test new methyl glucose derivatives for biodegradable wet wipes, non-irritating sunscreens, and edible films. A fair share of published papers focus on skin permeability and moisture retention, comparing methyl glucose against traditional polyols and noting comparable—or better—results in real-life testing. Brands invest in scaling up plant-based sourcing for increased consumer trust and reduced carbon impact. Researchers track every modification step for traceability, in part because of regulatory pressure, but also for competitive edge. Newer grades incorporate naturally derived fatty acid esters for improved texture and ease of use. Scientists continuously refine syntheses aiming for solvent-free and low-waste pathways, an effort that reduces regulatory hurdles and enhances product story in the crowded clean beauty arena.
Toxicology studies repeat the same lessons: methyl glucose barely registers as a hazard. Both acute and chronic testing in mammals report no mutagenicity or reproductive risks at realistic use concentrations. Eye and skin sensitization studies show rare transient irritation, often only at levels far above those found in consumer products. As I’ve seen in regulatory reviews, validated test data still swings the conversation—if a supplier can't provide the full stack of acute, subchronic, and cumulative studies, regulatory teams quickly move on. In aquatic toxicity panels, methyl glucose breaks down steadily and avoids long-term accumulation, another win for sustainability certifications. Ingredient houses keep a close watch on cross-contamination risks and prioritize non-animal test models where possible for further reassurance.
Methyl glucose remains on solid footing as demand grows for greener, safer, and more functional raw materials. Brands used to worry mostly about cost and quick blending, but now the questions run toward allergenicity, carbon footprint, and microplastic replacement. With continued advances in fermentation from non-GMO plant stocks, suppliers anticipate further price drops and less waste per kilo produced. The shift toward solid-format beauty and waterless formulations places methyl glucose at an advantage: it keeps performance even where some classic polymers and humectants fall short. As digital batch recordkeeping and machine learning reach ingredient plants, imagine even tighter tolerances and more transparent supply chains. In the next five years, new biodegradable methyl glucose blends for baby care, wound treatments, and even advanced food coatings look likely to move into commercial pipelines, backed by robust user data and better methods for scaling up without harsh reagents. Manufacturers see no slowdown, only new spaces to grow.
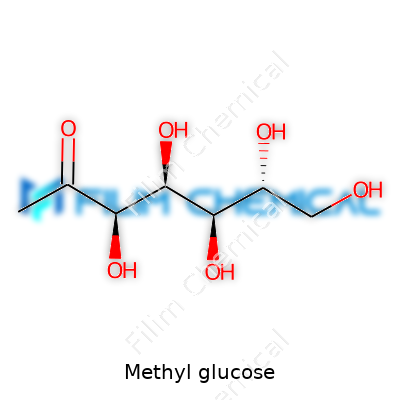
Methyl glucose might sound like a mouthful, but it's a small tweak on a simple sugar. Picture the glucose found in fruits, then attach a methyl group—a little molecular edit that changes how the sugar behaves. This shift gives methyl glucose new properties, setting it apart from plain table sugar or corn syrup.
Walk down the aisle of any supermarket, reach for a face cream, or stir your coffee. Methyl glucose and its relatives, like methyl glucoside or methyl glucose ether, often slip into ingredient lists. In skin lotions and hair products, methyl glucose derivatives add moisture, improve feel, and stabilize thick formulas. They leave skin smooth without a greasy finish. In shampoos, these ingredients help hair feel softer and less tangled.
Shifting to food, methyl glucose pops up as a minor player. Some food manufacturers turn to it for its ability to sweeten gently, but it usually doesn’t steal the spotlight from bigger names like sucrose. Its main fame stays in cosmetics and personal care, where it’s both gentle and practical.
People care about the stuff they put on their skin, especially as ingredients in lotions and shampoos drift into lakes and rivers. Safety studies give methyl glucose a green light. Studies listed by the Cosmetic Ingredient Review and FDA show that methyl glucose breaks down easily and doesn’t pile up in the environment. Its origin from a natural sugar also appeals to those steering away from petroleum-based chemicals.
Allergies and irritation come up a lot when people talk about skincare. Here’s where methyl glucose stands out. It has a low risk for causing rashes, making it a good choice for products aimed at sensitive or baby skin. Of course, no ingredient is perfect for everyone, but most people skate by without trouble.
The hunt for sustainable, plant-based alternatives is more than a trend. Manufacturers need solutions that deliver results without inviting backlash. Methyl glucose fits here because it supports texture and moisture—two deal breakers in lotions and hair products—without costing the earth or irritating skin.
I’ve tried dozens of brands hands-on as a researcher and as someone with classic dry winter skin. The switch from synthetic emollients to products using plant-based methyl glucose did what I wanted: my hands stayed hydrated longer, and the back of my neck stopped itching after shampoo. The feel makes a difference. People notice smoothness not just because it stays on the surface, but because it actually helps hold water inside the skin.
The move to greener ingredients comes with responsibility. Brands use methyl glucose to label their products as “safer” or “cleaner,” but transparency is key. More accurate labeling, regulatory oversight, and better public education will help consumers decide what works for them, instead of chasing buzzwords. Independent product testing and sharing real-world results, not just marketing gloss, matter here.
Methyl glucose plays a quiet but growing role in making personal care better for users and the planet. It’s not a magic bullet, but it does show that a simple chemical tweak sometimes leads to products that feel and work better in the real world.
Pick up a bottle of lotion or shampoo and scan the ingredients. Chances are, methyl glucose or its cousins (like methyl gluceth-10 or methyl glucose sesquistearate) pop up. The name doesn’t give away much—sounds chemical, but glucose is just a simple sugar. The “methyl” bit refers to a common chemical tweak that keeps the molecule stable and useful in creams or conditioners.
Products that claim to nourish, strengthen, or hydrate often rely on science-backed substances. Methyl glucose is valued for its ability to hold water, soften texture, and help products spread smoothly. Cosmetic chemists don’t just toss anything in the beaker. The safety of an ingredient matters every step of the way. Methyl glucose and its derivatives stand in a sweet spot—literally. They’re related to plant sugars and work well with the skin’s natural moisture processes.
Dermatologists and toxicologists have studied methyl glucose since it first landed in personal care. The Cosmetic Ingredient Review panel, an independent group of researchers, checked available data and found no meaningful risk at the levels found in personal care items. The U.S. Food and Drug Administration and Health Canada classify these compounds as safe for topical use. Studies show low potential for irritation, even for sensitive skin types.
Standing in the drugstore, I search for a face wash that doesn’t leave me flaky, especially during winter. Formulas heavy on methyl glucose do the trick: less tightness, more comfort. After talking to friends with eczema or easily irked scalps, most report no issues from these ingredients. In almost every case where someone had a problem, the culprit turned out to be fragrance or harsher plant extracts, not methyl glucose.
People have real concerns about “mysterious” ingredients in their body products. The long chemical names raise red flags, but many forget that some of the most innocent substances on earth sound intimidating on a label. The skincare world thrives on transparency, yet fear often spreads faster than fact. Companies could do a better job explaining what methyl glucose actually does. Many forget to mention its roots—this is not derived from petroleum nor known to disrupt hormones, two points many consumers care about.
Skin safety isn’t just about a lack of side effects in the test tube. Good science includes ongoing surveillance. Sometimes rare people react to a “safe” ingredient for odd reasons specific to their biology. Those cases remind us that patch testing before slathering on a new cream makes sense, even for time-tested ingredients.
If someone is looking to avoid anything synthetic, homemade options or “green beauty” lines exist, but methyl glucose remains a gentle choice by nearly any dermatologist’s standards. Allergic reactions can happen to almost anything—avocados, oats, chamomile—so absolute guarantees are rare. Still, the evidence so far puts methyl glucose in the low-risk category.
Brands and product makers should speak in plain language. Explaining where ingredients come from and what purpose they serve helps people make smarter choices. Clear, no-nonsense information beats hype or “free from” labels with no substance behind them. Ingredients like methyl glucose have passed the tests that matter most in skin safety. If new information eventually surfaces, the scientific world stands ready to adjust.
Methyl glucose, a sugar-based ingredient, pops up in all sorts of shampoos, lotions, and creams. It gets used to help with texture and feel, so lotions aren’t too greasy or runny. Even folks who keep a close eye on their skincare routines often miss it on a label—mostly because it sounds fairly harmless and sweet. But anyone with allergies or sensitive skin knows even ordinary ingredients can stir up problems, so it’s worth looking closer.
Talking with dermatologists and reading public data, there aren’t many reports of people breaking out in hives or swelling from just methyl glucose. For most, this ingredient acts gently on the skin. A study published in the International Journal of Toxicology covered various sugar derivatives like methyl glucose and didn’t flag them as top skin irritants. Most people, including those with eczema or mild sensitivities, glide right through without trouble.
Still, nothing’s guaranteed. People can react to almost anything, especially if their immune system sees a new ingredient over and over. Patch testing can spot early signs of irritation—such as redness or itching. If a rash or burning appears right after starting a new product, skipping it and consulting a doctor makes sense.
Manufacturers love methyl glucose for its mild profile—no stinging or lingering residue. That doesn’t mean everyone gets off scot-free. Folks living with a history of contact dermatitis or multiple allergies sometimes report itching or a slight sting, especially if they apply a thick layer regularly. Skin breaks or damaged skin barriers (like after too much sun or aggressive exfoliating) can let even milder chemicals cause a surprise flare-up.
Personal experience with sensitive skin makes clear that “hypoallergenic” doesn’t always mean risk-free. Ingredients that seem calm enough tend to work as expected until skin gets angry from something else—overuse, sunburn, harsh cleansers. It’s a reminder to start slow with any unfamiliar formula.
A big factor in unexpected reactions comes from the mix of ingredients in a product. Methyl glucose itself rarely triggers allergies, but the other chemicals or fragrances often found alongside it can tip the scales. Product recalls in the past often come from contamination or poor preservation, not the main ingredient. Cross-contamination in factories where nut oils or other known allergens get processed alongside methyl glucose products can’t be ignored.
Staying safe boils down to reading labels carefully, especially for those with allergies or chronic skin issues. The Food and Drug Administration tracks reports on personal care ingredients and hasn’t put methyl glucose on the warning list. The American Academy of Dermatology points out that patch testing at home—using a dab of new cream on the inside of the wrist and waiting twenty-four hours—still ranks as the simplest way to catch subtle allergies early.
Health experts also advise changing only one part of a routine at a time. That way, if redness or bumps show up, the culprit’s easier to spot. With so many products sharing the same core ingredients, taking a cautious approach can save a lot of itching and guessing.
Many of us scan the back of a cosmetic or food package and stumble across “methyl glucose” in the ingredients. The name itself doesn’t scream either “all-natural” or “lab creation,” so it’s natural to wonder where it actually comes from. To really understand this, it helps to look at both the process and the reason companies use it in products.
Methyl glucose comes from glucose, which the body knows well, thanks to bread, rice, or fruit. Glucose gets its name from plant-based sources—sugar beets, corn, and sugarcane fill grocery bags every day. Glucose itself falls firmly on the side of natural. Methyl glucose, though, comes from a simple chemical tweak: methylation. That step happens in a lab. Chemists take natural glucose and react it with methanol to add a methyl group. The substance at the end sits just a short distance from the fields but makes an important stop at the factory.
Shopping for skincare or edible products with the “clean” label calls for real scrutiny. Many people chase after ingredients that start their life in nature, even if they go through a few extra steps before showing up in a bottle. Methyl glucose is a good example of a gray zone. It owes its roots to plants, yet carries a twist that gives it new properties, like being more resistant to breaking down and less sticky. Lab work offers a way to change texture, shelf life, or safety.
Some folks see this chemical process and feel wary. Questions about safety spring up because synthetic chemicals sometimes remind people of harmful additives. Yet, methyl glucose doesn’t share that fate. Multiple safety reviews (including those from regulatory agencies in Europe and the United States) put methyl glucose on their “generally recognized as safe” lists for use in both cosmetics and foods. It has a history of causing neither irritation nor allergic reactions for most people. For skin-contact products, this makes it an ingredient that chemists reach for again and again.
The current system runs on efficiency and demand. Manufacturers chase ingredients that deliver on texture, shelf life, or consumer perception. Glucose from corn or cane is cheap and abundant. Transforming it in a lab doesn’t make it less plant-based in origin, but it does move it into the semi-synthetic column. Not everyone knows where the line sits. Some brands now reach for non-GMO glucose, then document every change to showcase their commitment to transparency. Traceability has become currency—consumers reward companies who show every step of sourcing and processing.
Honest labeling helps. Clearly showing if methyl glucose comes from natural glucose, and explaining how the process works, builds trust. Third-party certifications like COSMOS or USDA Organic step in to set the bar for what “natural” means. They don’t just look at where something starts—they ask about the whole journey. If a shopper wants a product to stay as close to nature as possible, these labels offer reassurance that lab work hasn’t drifted too far from the fields.
Education matters, too. More conversations between scientists, regulators, brands, and shoppers lower the odds of misunderstandings. If consumers know that methyl glucose gets its start in nature and passes through a mild transformation, they may see it as a practical part of modern products rather than a mystery item. That knowledge shapes trust more than buzzwords ever could.
Looking at ingredient labels, even on gentle cleansers, methyl glucose pops up often. Chemists call it a “humectant,” which means it pulls water into the skin, a bit like glycerin. This ingredient comes from corn or other plant starches, broken down and linked with fatty acids. It looks like a clear, gooey liquid or a white powder before blending into formulas. Brands grab it for its water-loving powers and how it helps hold a product together.
Anyone with sensitive skin knows the drill: one wrong ingredient turns a morning into a red, stinging mess. Dermatologists remind us that sensitive skin reacts because its natural barrier isn’t strong enough. In my own circle, friends with eczema or allergies run the “patch test” routine every time they try a new cream. They look for labels marked “fragrance-free,” but they study the ingredient list closely, too. Subtle sources of irritation—harsh surfactants, drying alcohols—can sneak into even “soothing” blends.
Here’s where methyl glucose stands out. Studies and real-world use show this ingredient does not irritate the skin. Researchers test it for stinging, redness, and allergic reactions. Groups like the Cosmetic Ingredient Review (CIR) panel have checked these studies, giving a thumbs-up on safety when used as intended. No big outbreaks of rash or swelling reported from creams, washes, or lotions containing methyl glucose.
As a humectant, it draws in water to plump up dry skin, which is a big win for anyone dealing with flakiness or tightness. It doesn’t strip away oils—something harsh sulfates and some alcohols do. That gives formulas with methyl glucose a softer touch. In real life, using products with this ingredient leaves skin feeling smooth, less itchy, and more comfortable. I remember trying a facial moisturizer with methyl glucose during the winter—my face never looked tight or shiny with it.
Scientific reviews on methyl glucose and its derivatives show a strong record of safety. CIR experts point to tests on humans and animals, with no evidence of irritation or allergy when used in the usual percentages (up to 5 percent in most products). Unlike ingredients that clog pores or trigger breakouts, methyl glucose remains lightweight and non-greasy. Brands like CeraVe, Cetaphil, and Vanicream keep turning to it in new launches aimed at sensitive skin.
The European Commission also lists methyl glucose derivatives as safe without restrictions in cosmetic products. People concerned about animal testing may appreciate that plant-based origins make it compatible with vegan and cruelty-free formulas.
Sensitive skin care always has room to grow. Some people react to preservatives, emulsifiers, or even thickening agents, so manufacturers should keep testing every component alongside methyl glucose. Pairing it with calming extracts such as oat or allantoin makes a good combo for extra-reactive skin. The ultimate goal is a product with few, carefully chosen ingredients, each tested on sensitive volunteers, not just healthy skin.
For everyday use, products with methyl glucose bring relief for people fighting dryness or splotchy reactions. Customers deserve to see simple, transparent ingredient labels and clear information about safety. Methyl glucose can stick around in formulas for sensitive skin, making life a little more comfortable for those needing that extra dose of kindness in their daily routine.
| Names | |
| Preferred IUPAC name | 1-O-Methyl-D-glucopyranose |
| Other names |
2-Methylglucose Methyl-D-glucopyranoside Methyl α-D-glucopyranoside Methyl β-D-glucopyranoside Methylglucoside |
| Pronunciation | /ˈmɛθɪl ˈɡluːkoʊs/ |
| Identifiers | |
| CAS Number | [6484-39-5] |
| Beilstein Reference | 391188 |
| ChEBI | CHEBI:132459 |
| ChEMBL | CHEMBL59008 |
| ChemSpider | 50473 |
| DrugBank | DB14155 |
| ECHA InfoCard | ECHA InfoCard: 100.004.685 |
| EC Number | EC 3.2.1.147 |
| Gmelin Reference | 107110 |
| KEGG | C01653 |
| MeSH | D008747 |
| PubChem CID | 6917 |
| RTECS number | SL9300000 |
| UNII | 4R8J12L5Q6 |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID9030246 |
| Properties | |
| Chemical formula | C7H14O6 |
| Molar mass | 194.18 g/mol |
| Appearance | White powder |
| Odor | Odorless |
| Density | 1.24 g/cm3 |
| Solubility in water | soluble |
| log P | -1.21 |
| Acidity (pKa) | 12.08 |
| Basicity (pKb) | 6.07 |
| Magnetic susceptibility (χ) | -72.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.465 |
| Viscosity | 3000 - 4500 cP |
| Dipole moment | 4.81 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 389.4 J mol⁻¹ K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1275.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2801 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | A16AX01 |
| Hazards | |
| Main hazards | May cause mild skin and eye irritation |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | May cause respiratory irritation. |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | > 210°C |
| LD50 (median dose) | LD50 (median dose): Rat oral 16,000 mg/kg |
| NIOSH | GJ0 |
| PEL (Permissible) | PEL for Methyl glucose: Not established |
| IDLH (Immediate danger) | Not listed |
| Related compounds | |
| Related compounds |
Glucose Methyl α-D-glucopyranoside Methyl β-D-glucopyranoside Ethyl glucoside Alkyl glucosides |